Johnson And Johnson Vaccine Approval Fda / The Day - Fauci: Approval of Johnson & Johnson ... / In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s.
Johnson And Johnson Vaccine Approval Fda / The Day - Fauci: Approval of Johnson & Johnson ... / In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s.. The fda's panel of independent. Moderna's trial found some hints that vaccinated people were less likely to develop an infection without symptoms. The company plans to deliver 20 million doses in over 65 million americans have already been vaccinated and about 1.3 million doses are being administered across the country each day. Vials with a sticker reading. Plus, the johnson & johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, the associated press notes.
The assessment was made as part of the agency's process for approving the vaccine. An independent group of fda advisers is expected to recommend that the vaccine be authorized in a meeting friday. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and pfizer/biontech. But what does this step in the process really mean? The food and drug administration of the us has now said that johnson & johnson's one shot corona vaccine appears to be safe and effective in the trial.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

A committee is set to meet friday to consider whether the fda should authorize the johnson & johnson vaccine for emergency approval. Plus, the johnson & johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, the associated press notes. In a staff report released on wednesday, the federal agency found the vaccine to be safe, effective. The company plans to deliver 20 million doses in over 65 million americans have already been vaccinated and about 1.3 million doses are being administered across the country each day. Officials found the vaccine was 68% effective against the variant in brazil and 64% effective against the south african variant. The food and drug administration of the us has now said that johnson & johnson's one shot corona vaccine appears to be safe and effective in the trial. The assessment was made as part of the agency's process for approving the vaccine. Regulators wednesday that armed with that advice, fda is expected to make a final decision within days.
A committee is set to meet friday to consider whether the fda should authorize the johnson & johnson vaccine for emergency approval.
The food and drug administration of the us has now said that johnson & johnson's one shot corona vaccine appears to be safe and effective in the trial. The fda's panel of independent. The assessment was made as part of the agency's process for approving the vaccine. The assessment paves the way for the drug's emergency use in the us, with experts meeting on friday to decide whether to approve it. The company plans to deliver 20 million doses in over 65 million americans have already been vaccinated and about 1.3 million doses are being administered across the country each day. Plus, the johnson & johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, the associated press notes. A final meeting is scheduled for friday, after which the agency will decide. It works by using a genetically modified. Johnson & johnson initially publicized the 66 percent effectiveness last month in a press release but had not yet released results from the trial. Regulators wednesday that armed with that advice, fda is expected to make a final decision within days. Now, an fda panel of independent experts plans to meet on friday to decide whether to approve the shot. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. Officials found the vaccine was 68% effective against the variant in brazil and 64% effective against the south african variant.
Vials with a sticker reading. The vaccination drive has been slower than hoped, hampered by logistical. Johnson & johnson announced that @janssenglobal has submitted an application to the @us_fda requesting emergency use authorization of its the j&j vaccine is different from the pfizer and moderna vaccines. Moderna's trial found some hints that vaccinated people were less likely to develop an infection without symptoms. The assessment paves the way for the drug's emergency use in the us, with experts meeting on friday to decide whether to approve it.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj

Johnson & johnson initially publicized the 66 percent effectiveness last month in a press release but had not yet released results from the trial. Now, an fda panel of independent experts plans to meet on friday to decide whether to approve the shot. But what does this step in the process really mean? Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. Johnson & johnson announced that @janssenglobal has submitted an application to the @us_fda requesting emergency use authorization of its the j&j vaccine is different from the pfizer and moderna vaccines. An independent group of fda advisers is expected to recommend that the vaccine be authorized in a meeting friday. The assessment paves the way for the drug's emergency use in the us, with experts meeting on friday to decide whether to approve it. The assessment was made as part of the agency's process for approving the vaccine.
An independent group of fda advisers is expected to recommend that the vaccine be authorized in a meeting friday.
The assessment was made as part of the agency's process for approving the vaccine. When the fda grants an emergency use authorization. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Meanwhile on capitol hill, the senate moved forward with a process that would allow democrats to approve a relief bill without republican support. The agency's independent advisors are set to meet on. Now, an fda panel of independent experts plans to meet on friday to decide whether to approve the shot. Johnson and johnson is known for its longstanding commitment—and proven track record—when it comes to fighting emerging epidemics. Regulators wednesday that armed with that advice, fda is expected to make a final decision within days. It works by using a genetically modified. The food and drug administration of the us has now said that johnson & johnson's one shot corona vaccine appears to be safe and effective in the trial. Vials with a sticker reading. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and pfizer/biontech. A committee is set to meet friday to consider whether the fda should authorize the johnson & johnson vaccine for emergency approval.
On tuesday, johnson & johnson said it could hand over four million doses upon approval of the vaccine. And astrazeneca found that its. The assessment paves the way for the drug's emergency use in the us, with experts meeting on friday to decide whether to approve it. The fda's panel of independent. Plus, the johnson & johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, the associated press notes.
Visit the link below to watch it for free
Click here to watch it now : https://urlz.fr/eVmj
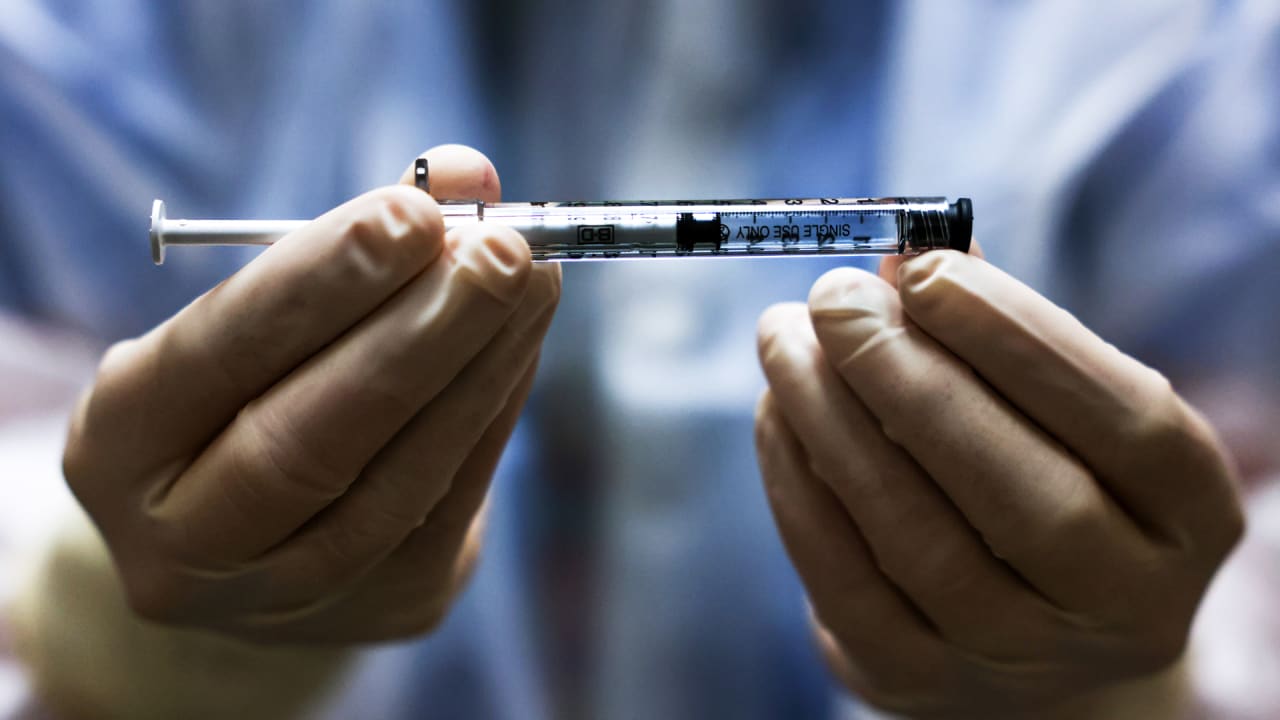
Regulators wednesday that armed with that advice, fda is expected to make a final decision within days. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and pfizer/biontech. It works by using a genetically modified. The company plans to deliver 20 million doses in over 65 million americans have already been vaccinated and about 1.3 million doses are being administered across the country each day. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. The fda's panel of independent. In a staff report released on wednesday, the federal agency found the vaccine to be safe, effective.
The johnson & johnson vaccine could be the third vaccine granted emergency use authorization by the fda, following vaccines made by moderna and pfizer/biontech.
When the fda grants an emergency use authorization. The agency's independent advisors are set to meet on. In congressional testimony tuesday, a johnson & johnson executive said 4 million doses of vaccine would be available in the u.s. Food and drug administration said wednesday, paving the way for its approval for emergency use as soon as this week. Now, an fda panel of independent experts plans to meet on friday to decide whether to approve the shot. Food and drug administration (fda) staff said in documents published on wednesday, paving the way for its approval for emergency use. The food and drug administration of the us has now said that johnson & johnson's one shot corona vaccine appears to be safe and effective in the trial. Johnson & johnson appears to be just as good as moderna and pfizer at preventing those, jha said. Officials found the vaccine was 68% effective against the variant in brazil and 64% effective against the south african variant. The vaccination drive has been slower than hoped, hampered by logistical. Plus, the johnson & johnson vaccine can also be stored for three months in a refrigerator, rather than having to be kept frozen, the associated press notes. Vials with a sticker reading. And astrazeneca found that its.
Komentar
Posting Komentar